|
Metal boryl complexes and their reactivity : Metal boryl complexes have been extensively studied because of their implication in catalytic borylation reactions. More specifically, the transfer of a metal boryl moiety to organic substrate has provided an efficient and facile route to boronate precursors in the formation of C‒C, C‒O and C‒N bonds via the act of cross-coupling reactions. In addition, metal boryl species have also been documented in the borylation of alkynes, alkenes, 1,3-dienes, α,β-unsaturated ketones, and allenes. Due to their applicability and synthetic importance, there are tremendous interest in the synthesis and isolation of metal boryl complexes given that these are proposed intermediates in many catalytic systems.
The metal boryl complexes can be synthesised from five general routes: (i) salt elimination: nucleophilic attack of anionic metal complexes on X‒BR2 (X = Cl, Br, I); (ii) the use of metal hydride with H‒BR2; (iii) σ-bond metathesis reactions, (iv) oxidative addition of X‒BR2, H‒BR2, and R2B‒BR2 across electron rich metal Centres and (v) metal alkoxy complexes mediate B‒B bond cleavage. Our main interest is extension of such chemistry to the d- and early f-block elements for the synthesis of metal boryl complexes and their reactivity.
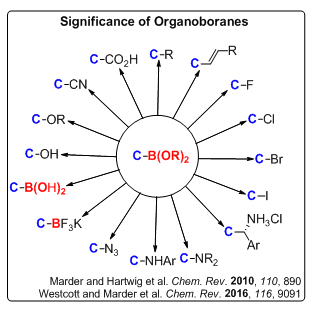
Catalytic Borylation: Organoboranes and boronic esters are important class of compounds in synthetic, material, biological, and medicinal chemistry. With chemical stability and synthetic versatility, these derivatives are attractive intermediates for constructing complex natural products or as synthetic building blocks in organic synthesis. In addition, some of these compounds possess important biological activities and are used as anticancer agents. Such a great importance has consistently stimulated the development of new synthetic routes to obtain target organoboron compounds more easily and efficiently. Over the last few years, considerable effort has been committed to their preparation using expensive and toxic precious metals (such as Pd, Rh, Ir, etc.). Recently, non-precious metal alternatives (e.g. Cu, Fe and even Zn) have emerged, which are of interest due to their low cost, toxicity and environmental advantages. We intend to develop non-precious metal catalyst system for C-B and C-X bonds formation reactions.
 |
Our foremost research areas:
- Homogeneous catalysis: Transition metal-catalyzed C-B bond formation reactions
- Borylation reactions catalyzed by metal nanoparticles
- Synthesis of metal boryl (mono and bimetallic) complexes and their reactivity
- Organometallic Chemistry: Synthesis of compounds featuring metal - (main group element) multiple bonds.
|
|



